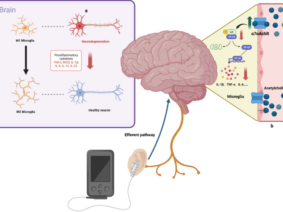
Vagus nerve stimulation (VNS) has been used for nearly 20 years in the treatment of diseases such as epilepsy and depression and is duly approved by the US Food and Drug Administration. The vagus nerve has been known to play a role in maintaining homeostasis, controlling stress, and regulating the immune system. VNS can be performed electrically and invasively from the neck, but non-invasively from the neck or ear. Due to the variable character of the autonomic nervous system activity, it is seen that the application of personalized current parameters is more important in VNS compared to other neuromodulation methods. In order to increase the effectiveness of the treatment and reduce possible side effects, stimulation parameters (frequency, duration, pulse width, etc.) can be shaped online with closed-loop stimulation according to patient data. In order to create personalized stimulation parameters, measurements such as electroencephalography, functional magnetic resonance imaging, heart rate variability, pulse, blood pressure, evoked potential can be collected continuously from the individual. Stimulation parameters can be determined more clearly, thanks to the follow-up of the effects on the individual as a result of the stimulation.
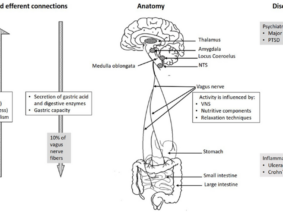
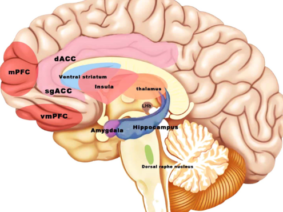
Vagus nerve stimulation (VNS) is a treatment method approved by the FDA (Food and Drug Administration) for the treatment of resistant epilepsy since 1997 and also for treating resistant depression since 2005. The vagus nerve is the most important and largest part of the parasympathetic nervous system. This nerve which has nuclei in the brain stem descends from the neck and makes connections with almost all internal organs (1). This method, which starts with the stimulation of the cervical vagus nerve, is often performed invasively by placing a programmed electrical generator on the chest wall and is unilateral (2). Apart from its role in epilepsy and depression diseases, the vagus nerve provides the brain-intestinal connection and is also responsible for establishing homeostasis. Vagus nerve stimulation can also affect the immune system through the cholinergic anti-inflammatory pathway. The method has shown promising results in the treatment of chronic inflammatory disorders such as inflammatory diseases of the digestive tract, sepsis, lung injury, rheumatoid arthritis, and diabetes. It is also used to control pain in fibromyalgia and migraine (3,4,5). Stimulation of vagal afferent fibers affects the monoaminergic system in the brain stem, which plays an important role in major psychiatric conditions such as mood and anxiety disorders. The vagus nerve plays an important role in the regulation of nutrition, satiety, and energy homeostasis. Stress can trigger the sympathetic system and decrease vagal tone and result in impairment of the mentioned functions. The vagus nerve has connections with brain regions (locus coeruleus, orbitofrontal cortex, insula, hippocampus, and amygdala) that are activated under stress. VSU can reduce the effects of stress on the body by modulating the activity of the vagus nerve (6). The connections of the vagus nerve with the cardiovascular system form the basis of the use of vagus nerve stimulation in diseases such as heart failure, arrhythmias, ischemia, and infarction. The high-frequency component of heart rate variability is considered an indicator of vagal tone (7). Vagus nerve stimulation is a method with indications and potential for use in diverse diseases and disorders, as studies have indicated.
Vagus Nerve Stimulation Methods
In addition to cervical invasive stimulation methods, vagus nerve stimulation can be performed by cervical noninvasive and auricular transcutaneous or percutaneous methods. Electrical stimulation is the main form of stimulation. In addition to its wide distribution in the body, VNS can be used in many different indications thanks to the connections of its nuclei in the brain stem (8). Some of the invasive VNS devices have a closed-loop system and include a cardiac-based seizure detection algorithm. Automatic stimulation is provided by triggering the heart rate increase due to the seizure. However, the optimal stimulation parameters are still unclear. Invasive VNS requires surgery; however surgical complications, side effects caused by stimulation of efferent fibers, and its cost limit its use (9). Although invasive VNS is clinically beneficial in 60% of drug-resistant epilepsy patients, side effects such as hoarseness, sore throat, shortness of breath and cough are common. Auricular Vagus Nerve Stimulation, as a method targeting the ear branch of the vagus, can activate similar neuronal systems in the brain, similar to invasive stimulation. The projection of vagus nerve ear afferents to the nucleus tractus solitarius is known from histochemical and electrophysiological experiments (10). When the existing literature is examined, it is seen that there is no clear consensus on the regions where the vagus nerve is most concentrated in the ear. However, it is reasonable to point out that the inner part of the concha and tragus are suitable sites for vagal modulation. When evaluated in terms of therapeutic efficacy, there is no consensus in the literature about the optimum parameters of auricular VNS, similar to invasive stimulation (11). In different situations; it remains unclear what the wavelength, intensity, and frequency of the excitation should be. A systematic approach is required to determine the optimal stimulation intensity, pulse width, waveform, and frequency that confers the greatest clinical benefit. This may require setting participant-specific parameters in a closed-loop setup where stimulation parameters are set online. All current stimulation strategies for noninvasive VNS devices are based on open-loop control of stimulation parameters, where levels are set at the start of the stimulation protocol and do not change in response to any continuous measurement of neuronal activation level. It is reasonable to expect different results in response to open-loop electrical stimulation between participants and between trials, due to the different brain activities that go on during stimulation. In a system in which personalized stimulation parameters are shaped according to the collected data, it can be expected that treatment efficacy will increase and possible side effects will decrease. With VNS, a system in which changes in the organism are constantly measured should be targeted. Measurements such as electroencephalography, functional magnetic resonance imaging, heart rate variability, pulse, blood pressure, evoked potential; can be collected both during stimulation and during non-stimulation (to determine the most appropriate treatment time). Collecting data outside the stimulation time can also help to understand how long the stimulation’s effectiveness lasts after the stimulation ends. Many studies have shown that Vagus Nerve Stimulation participants who complete their treatment throughout the study respond better than those who quit halfway. Also, it was noted that longer treatment durations correspond to better therapeutic outcomes. The mechanism of VNS is still not fully clarified. A closed-loop stimulation method can help us better understand the functioning of the autonomic nervous system. An effective and safe method, common side effects with auricular VNS include tingling or pain around the stimulation site; some participants reported itching or redness. Although it is an easily applicable form of treatment with few side effects, finding the right stimulation parameters for each disease and patient remains a critical challenge in auricular VNS. Clinicians should customize treatment for different functional conditions (12,13).
Lateral or Bilateral Auricular Vagus Nerve Stimulation
Another issue in Auricular Vagus Nerve Stimulation is about which ear the stimulation should be made from. Invasive cervical VNS is done from the left side to reduce cardiac side effects because it also stimulates efferent fibers. For this reason, auricular VNS is similarly often performed on the left side. However, stimuli from the right or left ear eventually go to the same place, the nucleus tractus solitarius. Simultaneous activation of the left and right auricular vagus nerves can potentially increase stimulation effects due to increased sensory input to the brainstem (14). There are very few documented studies in the literature in which bilateral auricular VNS is performed (15). Using artificial intelligence through sensors, it may be possible in the near future to decide whether stimulation should be done in the right, left, or both ears.
Stimulation Optimization
Due to the complex physiology of the body, continuous and intermittent stimulation, as well as strong or moderate stimulation, can even cause opposite physiological effects. Certain stimulation patterns may cause compensatory increased sympathetic activity along with the increased parasympathetic activity. The easiest aspect of providing closed-loop stimulation is to obtain subjective data from the person being administered. However, the absence of objective physiological data may result in the inaccurate or incomplete evaluation of treatment efficacy. Correct selection and processing of physiological signals as recorded by the sensor or sensors is crucial for closed-loop auricular vagus nerve stimulation because the feedback must include information about the characteristics of physiological reactions occurring in response to treatment. Auricular vagus nerve stimulation systems with closed-loop or biofeedback can be integrated with telemedicine applications (16). vagus nerve; It is a complex nerve that provides afferent and efferent innervation of the pharynx, larynx, heart, lungs, esophagus, stomach, liver, pancreas, small intestine, and proximal colon. This widespread distribution allows vagus nerve stimulation to have an effect over a wide window. Since cervical vagus nerve stimulation also includes efferent fibers, selective stimulation of these fibers may prevent off-target effects (17,18). In auricular stimulation, on the other hand, different stimulation points may cause different effects (11). In vagus nerve stimulation, a personalized treatment program is very important, as in other areas of medicine. Based on the results of clinical studies in animals and humans, an experimental idea about the ideal stimulation can be obtained. In the light of these results, treatment guidelines can be created (19). However, it can be expected that a closed-loop stimulation, in which the activity of the vagus nerve is personally recorded, subjective and objective data are collected through sensors, the data is processed, and the optimal stimulation parameters are determined with artificial intelligence (20). Although there is currently no medical device that can do all of these features, there are devices and clinical studies in which the sensor-stimulation algorithm is used as a precursor (21,22,23). New method proposals for bidirectional neuromodulation, in which simultaneous recording and stimulation are performed on the same nerve, signal processing, and system control on an external computer, are also encountered in the literature (24). Although vagus nerve stimulation is a method that prioritizes regulation in the autonomic nervous system due to its nature, it was found that the treatment led to different results in patients particularly in evaluations based on heart rate and heart rate variability (25). From this, it can be deduced as a result of the diversification of the data to be collected from the people who have VNS. Somatosensory evoked potential, pupil diameter, and salivary alpha-amylase levels can be used to evaluate VNS activity (26). In noninvasive methods, it may also be necessary to quantify vagus nerve stimulation (27).
Vagus Nerve Stimulation Methods
Although closed-loop stimulation systems are used for conditions such as Parkinson’s disease or pain other than epilepsy; it can be stated that the number of available devices, the type of sensors, and data used is low (28). The automatic stimulation feature triggered by an increase in ictal heart rate by at least 20% is used to transmit vagus nerve stimulation in a closed-loop manner in epilepsy patients (21). A closed-loop VNS method in which the device remains on-off according to heart rate in a heart failure-induced sheep Ugalde et al. defined by (29). A closed-loop stimulation according to heart rate to the cervical vagus nerve of pigs under anesthesia was successfully performed invasively and stabilization of heart rate under VNS was achieved (30). Different VNS closed-loop control systems are also proposed for real-time regulation of heart rate. In the proposed model, multiple VNS parameters can be modulated according to the applied object and the model can be integrated into implanted devices (31,32). In the study of Cork et al., the extracellular pH in the subdiaphragmatic vagus nerve of rats under anesthesia was measured and it was stated that this method could be used in closed-loop VNS (33). Diffusion tensor imaging and machine learning algorithms can be used to select patients with epilepsy who are more likely to benefit from VNS therapy outside the closed-loop (34). Vagus nerve stimulation has emerged as a method that can be used in the treatment of different ailments, can be applied invasively or non-invasively, and has gained importance in recent years. Recently, treatment approaches that include closed-loop algorithms and artificial intelligence in medicine have also started to come to the fore. It can be expected that the success rate of VNS treatment will increase and its use will become widespread in conditions were noninvasive, easy to apply, personalized stimulation parameters, and detailed follow-up with data collected from individuals.
References
- Ohemeng KK, Parham K. Vagal Nerve Stimulation: Indications, Implantation, and Outcomes. Otolaryngol Clin North Am. 2020 Feb;53(1):127-143.
- Ekmekçi H, Kaptan H. Vagus Nerve Stimulation. Open Access Maced J MedSci. 2017 May 7;5(3):391-394.
- Bonaz B, Sinniger V, Pellissier S. Vagus Nerve Stimulation at the Interface of Brain–Gut Interactions. Cold Spring Harb Perspect Med. 2019 Aug 1;9(8):a034199.
- Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016 Oct 15;594(20):5781-5790.
- Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 2018 May 16;11:203-213.
- Breit S, Kupferberg A, Rogler G, Hasler G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry. 2018 Mar 13;9:44.
- Capilupi MJ, Kerath SM, Becker LB. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb Perspect Med. 2020 Feb 3;10(2):a034173.
- Farmer AD, Albu-Soda A, Aziz Q. Vagus nerve stimulation in clinical practice. Br J Hosp Med (Lond). 2016 Nov 2;77(11):645-651.
- Mertens A, Raedt R, Gadeyne S, Carrette E, Boon P, Vonck K. Recent advances in devices for vagus nerve stimulation. Expert Rev Med Devices. 2018 Aug;15(8):527-539.
- Ellrich J. Transcutaneous Auricular Vagus Nerve Stimulation. J Clin Neurophysiol. 2019 Nov;36(6):437-442.
- Butt MF, Albusoda A, Farmer AD, Aziz Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat. 2020 Apr;236(4):588-611.
- Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front Neurosci. 2020 Apr28;14:284.
- Wang Y, Li SY, WangD, et al. Transcutaneous Auricular Vagus Nerve Stimulation: From Concept to Application. Neurosci Bull. 2020 Dec 23. DOI: 10.1007/s12264-020-00619-y.
- Kaniusas E, Kampusch S, Tittgemeyer M, et al. Current Directions in the Auricular Vagus Nerve Stimulation I – A Physiological Perspective. Front Neurosci. 2019 Aug9;13:854.
- Kutlu N, Özden AV, Alptekin HK, Alptekin JÖ. The Impact of Auricular Vagus Nerve Stimulation on Pain and Life Quality in Patients with Fibromyalgia Syndrome. Biomed Res Int. 2020 Feb28;2020:8656218.
- Kaniusas E, Kampusch S, Tittgemeyer M, et al.Current Directions in the Auricular Vagus Nerve Stimulation II – An Engineering Perspective. Front Neurosci. 2019 Jul 24;13:772.
- Thompson N, Mastitskaya S, Holder D. Avoiding off-target effects in electrical stimulation of the cervical vagus nerve: Neuroanatomical tracing techniques to study fascicular anatomy of the vagus nerve. J Neurosci Methods. 2019 Sep1;325:108325.
- Plachta DT, Gierthmuehlen M, Cota O,et al. Blood pressure control with selective vagal nerve stimulation and minimal side effects. J NeuralEng. 2014 Jun;11(3):036011.
- Musselman ED, Pelot NA, Grill WM. Empirically Based Guidelines for Selecting Vagus Nerve Stimulation Parameters in Epilepsy and Heart Failure. Cold Spring Harb Perspect Med. 2019 Jul 1;9(7):a034264.
- Guiraud D, Andreu D, Bonnet S, et al.Vagus nerve stimulation: state of the art of stimulation and recording strategies to address autonomic function neuromodulation. J Neural Eng. 2016 Aug;13(4):041002.
- Boon P, Vonck K, van Rijckevorsel K, et al. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure. 2015 Nov;32:52-61.
- Cukiert A, Cukiert CM, Mariani PP, Burattini JA. Impact of Cardiac-Based Vagus Nerve Stimulation Closed-Loop Stimulation on the Seizure Outcome of Patients With Generalized Epilepsy: A Prospective, Individual-Control Study. Neuromodulation. 2020 Oct 12. doi: 10.1111/ner.13290.
- Tzadok M, Harush A, Nissenkorn A, Zauberman Y, Feldman Z, Ben-Zeev B. Clinical outcomes of closed-loop vagal nerve stimulation in patients with refractory epilepsy. Seizure. 2019 Oct;71:140-144.
- Xu J, Guo H, Nguyen AT, Lim H, Yang Z. A Bidirectional Neuromodulation Technology for Nerve Recording and Stimulation. Micromachines (Basel). 2018 Oct 23;9(11):538.
- Anand IS, Konstam MA, KleinHU, et al. Comparison of symptomatic and functional responses to vagus nerve stimulation in ANTHEM-HF, INOVATE-HF, and NECTAR-HF. ESC Heart Fail. 2020 Feb;7(1):75-83.
- Burger AM, D’Agostini M, Verkuil B, Van Diest I. Moving beyond belief: A narrative review of potential biomarkers for transcutaneous vagus nerve stimulation. Psychophysiology. 2020 Jun;57(6):e13571.
- Mourdoukoutas AP, Truong DQ, Adair DK, Simon BJ, Bikson M. High-resolution Multi-Scale Computational Model for Non-invasive Cervical Vagus Nerve Stimulation. Neuromodulation. 2018 Apr;21(3):261-268.
- Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014 Jul;11(3):553-63.
- Ugalde HR, Le Rolle V, Bel A,et al. On-off closed-loop control of vagus nerve stimulation for the adaptation of heart rate. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:6262-5.
- Tosato M, Yoshida K, Toft E, Nekrasas V, Struijk JJ. Closed-loop control of the heart rate by electrical stimulation of the vagus nerve. Med Biol Eng Comput. 2006 Mar;44(3):161-9.
- Romero-Ugalde HM, Le Rolle V, BonnetJL, et al. A novel controller based on state-transition models for closed-loop vagus nerve stimulation: Application to heart rate regulation. PLoSOne. 2017 Oct 27;12(10):e0186068.
- Romero-Ugalde HM, Le Rolle V, Bonnet JL, Henry C, Mabo P, Carrault G, Hernandez AI. Closed-loop vagus nerve stimulation based on state transition models. IEEE Trans Biomed Eng. 2018 Jul;65(7):1630-1638.
- Cork SC, Eftekhar A, Mirza KB, et al. Extracellular pH monitoring for use in closed-loop vagus nerve stimulation. J NeuralEng. 2018 Feb;15(1):016001.
- Mithani K, Mikhail M, Morgan BR, et al. Connectomic Profiling Identifies Responders to Vagus Nerve Stimulation. Ann Neurol. 2019 Nov;86(5):743-753.